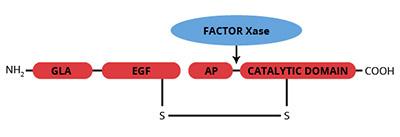
Factor X is a vitamin K-dependent protein zymogen which is synthesized in the liver and circulates in plasma as a two chain molecule linked by a disulfide bond (1,2). Prior to secretion into plasma, post-translational modifications produce 11 gamma-carboxyglutamic acid (gla) residues and a single b-hydroxyaspartic acid residue, which are located within the NH2-terminal light chain. The light chain also contains two epidermal growth factor (EGF) homology domains. The COOH-terminal heavy chain of factor X contains most of the carbohydrate moieties, as well as the latent serine protease domain. The activation of factor X is catalyzed by either the intrinsic factor Xase complex (factor IXa, factor VIIIa, cellular surface and calcium ions) or the extrinsic factor Xase complex (factor VIIa, tissue factor, cellular surface and calcium ions). Activation of human factor X by either complex results in cleavage at Arg52-Ile53 of the COOH-terminal heavy chain and subsequent release of a 52 amino acid activation glycopeptide. Factor Xa then serves as the enzyme component of the prothrombinase complex which is responsible for the rapid conversion of prothrombin to thrombin. The gla residues enable factor X/Xa to bind phospholipid (i.e. cell surfaces) in a calcium dependent manner; a requirement for assembly of the prothrombinase complex. The first EGF homology domain contains a Ca2+ binding site which acts as a hinge to fold the EGF and GLA domains towards each other (12). This region of the molecule is involved in the recognition of cellular binding domains.
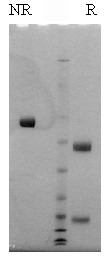
Human factor X is isolated from fresh frozen human plasma by a combination of conventional techniques (3) and immunoaffinity chromatography (4). In addition to the standard human factor X preparation, Gla-domainless human factor X is also available. Bovine factor X is isolated from fresh bovine plasma using a modification of the procedure reported by Bajaj et al. (5,6). The purified zymogen is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20oC. Purity is determined by SDS-PAGE analysis and activity is measured in a factor X clotting assay.