Activation of the zymogen, factor X, by either the intrinsic or extrinsic factor Xase complexes produces the active serine protease factor Xa (1,2). The activation of factor X requires proteolytic cleavage of the heavy chain, resulting in the release of an activation glycopeptide. The heavy chain region in factor Xa contains the serine protease catalytic domain, while the light chain, as in the zymogen, contains the membrane binding domain.
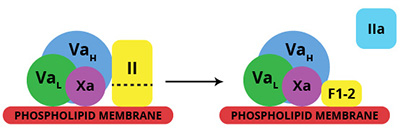
Factor Xa (molecular weight 46,000) participates in the prothrombinase complex, which catalyzes the rapid conversion of prothrombin to thrombin. Prothrombinase is an enzyme complex composed of factor Xa (enzyme) and factor Va (cofactor) assembled on a cellular surface in the presence of calcium ions. Although factor Xa can independently catalyze the activation of prothrombin, the rate at which this reaction occurs is increased nearly 300,000-fold with complete assembly of the prothrombinase complex. The clotting activity of factor Xa in vivo is terminated by either inactivation of the cofactor, factor Va, or by direct inhibition of factor Xa by inhibitors, such as ATIII, after disassembly of the prothrombinase complex.
In addition to its broad application in coagulation research, factor Xa can be utilized for site specific cleavage of fusion proteins expressed in bacteria (9-12). A factor Xa-sensitive site is incorporated between the recombinant protein of interest and peptides or proteins which facilitate purification and/or expression. The target protein is released from the expressed hybrid by cleavage with factor Xa. The factor Xa can then be easily removed by affinity chromatography.
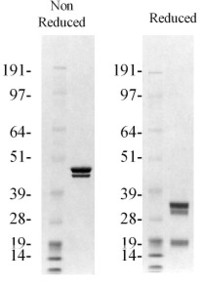
Factor Xa is prepared by activating purified factor X with the factor X activator isolated from Russell’s viper venom. Factor Xa is purified from the activation mixture by chromatography over benzamidine-Sepharose followed by gel filtration (1,3). Several modified forms of factor Xa are also available including: A) active-site blocked factor Xa containing either the tripeptide chloromethyl ketone inhibitor EGRck, or the fluorescent inhibitor Dansyl-EGRck; and B) human Gla-domainless β-factor Xa. The enzyme is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20°C. Purity is determined by SDS-PAGE analysis and activity is measured in a factor Xa clotting assay and/or chromogenic substrate assay. Lot to lot consistency ensures reproducible results every time.
Cell culture: For experiments involving cell cultures, please contact us to discuss custom, low endotoxin lots designated for cell culture use.