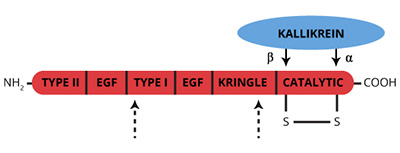
Factor XII (XII) (Hageman Factor) is a single chain (Mr=78,000) glycoprotein zymogen that circulates in plasma at a concentration of 40 µg/ml (1-5). Reciprical activation of XII to the active serine protease factor XIIa (XIIa) by kallikrein is central to initiation of the intrinsic coagulation pathway. Surface bound α-XIIa in turn activates factor XI to XIa. Secondary cleavage of α-XIIa by kallikrein yields β-XIIa, which catalyzes solution phase activation of kallikrein, factor VII and the classical complement cascade.
The ability of a variety of negatively charged substances, both physiological and nonphysiological to promote XII activation and, thus, initiation of the intrinsic pathway has led to the psuedonym “contact activation”. Binding to anionic surfaces induces a conformational change, making the XII zymogen more susceptible to cleavage by a variety of proteases (6,7). It is unlikely that binding to negatively charged surfaces alone is sufficient to activate XII, since highly purified preparations of XII and plasma deficient in prekallikrein and high molecular weight kininogen do not undergo this “autocatalysis” (8-11).
A single cleavage by kallikrein at R353-Val354 of XII yields α-XIIa, a 2 chain protease (Mr=80,000) held together by disulfide bonds. The COOH-terminal light chain (Mr=28,000) contains the catalytic triad (His-40, Asp-89, Ser-191), while the NH2-terminal heavy chain (Mr=52,000) conatins the anionic surface binding portion of the molecule. A secondary cleavage of α-XIIa by kallikrein outside the disulfide bond yields β-XIIa (XIIf, BHFa, HFf, hageman factor fragments) (Mr=28,000), which no longer binds anionic surfaces (12). β-XIIa can activate prekallikrein, but has little procoagulant activity (13,14). Several other minor intermediate forms of XIIa are indicated in the figure above.
Inhibitors of XIIa include C1-INH, α2-antiplasmin, α2-macroglobulin and antithrombin III. At physiological concentrations, the relative effectiveness of these inhibitors is 91 : 4.5 : 3 : 1.5, respectively (10, 16-19). The ratio of C1-INH to XII has been implicated in the “cold activation” of factor VII and the conversion of prorenin to renin on storage of plasma (20,21).
Human factor XII is prepared from fresh frozen plasma by immunoaffinity chromatography and supplied in 50% glycerol/4mM Sodium Acetate, 0.15M NaCl, pH 5.3 for storage at -20oC.