Protein S is a single chain vitamin K-dependent protein which is thought to function in both the coagulation and complement cascades (1,2). Approximately 60% of protein S circulating in plasma is complexed to C4b binding protein (C4BP). It has been suggested that g-carboxyglutamic acid (gla) dependent binding of protein S to negatively charged phospholipids may function to concentrate C4BP at cell surfaces following injury.
In the coagulation system, protein S functions as an anticoagulant cofactor protein. Activated protein C (APC) forms a 1:1 stoichiometric complex with protein S in the presence of Ca2+ and phospholipid vesicles (Kd=6×10-9M) (3). In the presence of protein S, a moderate increase (3-10 fold) in the rate of factor Va and factor VIIIa inactivation by APC is observed in plasma and on the surface of unstimulated platelets. Protein S bound to C4BP does not possess APC cofactor activity. Recently, an additional binding protein which enhances the activity of protein S has been described (4). Proteolytic inactivation of protein S by thrombin has been proposed as a regulatory mechanism in this system. A single cleavage by thrombin abolishes protein S cofactor activity by removing an NH2-terminal peptide (Mr=8000) which contains the gla domain.
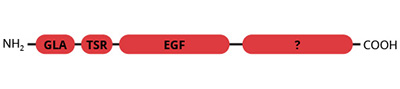
The domain structure of protein S is similar to that of the other vitamin K-dependant coagulation factors with the exception that protein S does not possess the catalytic triad. Protein S is a single chain protein containing 10 gla residues in the NH2-terminal domain and 4 epidermal growth factor (EGF) domains.
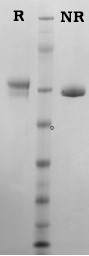
Human protein S is isolated from fresh frozen plasma by a combination of conventional methods (9) and immunoaffinity chromatography as described by Jenny et al. (5). Purified protein S is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20°C. Purity is determined by SDS-PAGE analysis.