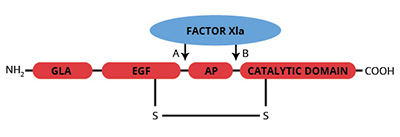
The zymogen factor IX is a single chain vitamin K-dependent glycoprotein which is synthesized in the liver (1-3). The domain structure of factor IX is similar to that of the other vitamin K dependent coagulation factors. The NH2-terminal region contains 12 γ-carboxyglutamic acid (gla) residues which facilitate the calcium dependent binding of factor IX to negatively charged phospholipid surfaces. Two domains which are homologous to epidermal growth factor (EGF) span the region between the NH2-terminal gla domain and the activation peptide (Ala-146 to Arg-180).
Factor IX is activated by either factor XIa or the factor VIIa/tissue factor/phospholipid complex. Cleavage at site A (see figure) yields the intermediate IXa which is subsequently converted to the fully active form IXaβ by cleavage at site B. The NH2-terminal light chain (GLA and EGF domains) remains covalently attached to the COOH-terminal heavy chain by a disulfide bond. The serine protease catalytic triad (Ser-365, His 221, Asp-269) is located in the heavy chain. Factor IXaβ is the catalytic component of the “intrinsic factor Xase complex” (factor VIIIa/IXa/Ca2+/phospholipid) which proteolytically activates factor X to factor Xa.
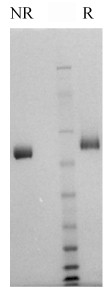
Human factor IX is prepared from fresh frozen plasma by a combination of conventional procedures (4) and immunoaffinity chromatography (5). Bovine factor IX is prepared from fresh citrated bovine plasma by a modification of the method described by Fujikawa et al. (6). The purified proteins are supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20oC. Purity is determined by SDS-PAGE analysis and activity is measured using a factor IX clotting assay.