Activated protein C (APC) is an anticoagulant serine protease derived from the two chain, vitamin K-dependent zymogen, protein C (3-7). A complex between alpha-thrombin and thrombomodulin catalyzes a single cleavage at Arg-12 (Arg-14 in bovine) in the heavy chain of protein C, to generate activated Protein C. Several non-physiologically relevant proteases such as RVV-X activator, trypsin, and PROTAC are also capable of activating protein C.
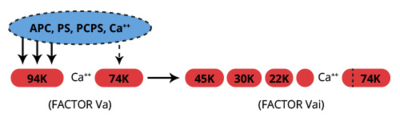
APC functions as an anticoagulant which catalyzes the proteolytic inactivation of the cofactors, factors Va and VIIIa, leading to inhibition of the prothrombinase and factor Xase complexes. The inactivation of factors Va and VIIIa is both Ca2+ and phospholipid dependent. The vitamin K dependent cofactor, protein S, moderately increases this rate of inactivation by forming a 1:1 complex with APC (Kd=6×10-9M) (8).
Several factors attenuate the anticoagulant activity of APC. Factor Xa protects factor Va from proteolysis by APC by competing for a similar binding site on factor Va. Thrombin has also been proposed as a regulator of APC by proteolytic inactivation of protein S. In addition, APC is regulated by a circulating heparin-dependent protein C inhibitor (PAI-3), a circulating heparin-independent protein C inhibitor, a platelet-derived protein C inhibitor, and PAI-1. The complexes formed between APC and both types of PAI have been reported to account for increased fibrinolysis observed upon infusion of APC or the generation of APC in vivo.
In addition to our standard APC preparation, an active site-blocked form containing Dansyl-EGR-chloromethlyketone is also available.
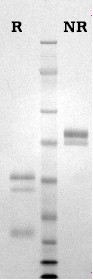
Activated protein C is prepared from purified protein C by activation with thrombin followed by ion exchange chromatography (4). APC is supplied in 50% (vol/vol) glycerol/H2O and should be stored at -20oC. Purity is determined by SDS-PAGE analysis and activity is measured using a chromogenic substrate assay. All production lots of APC are also tested for their ability to prolong the aPTT of normal human plasma, as required for the APC resistance assay (10,11). The results of this test are provided for each lot, as an aPTT (+/- APC) ratio (10nM APC).